Cytokinetics Presents Additional Data From GALACTIC-HF at the American Heart Association Scientific Sessions 2024
“These analyses reinforce the potential treatment benefit of omecamtiv mecarbil in patients from GALACTIC-HF who are at higher risk, such as older patients or those with a recent ventricular arrhythmia,” said
Treatment with Omecamtiv Mecarbil Reduced Risk of the Primary Composite Endpoint in Patients with Severe Heart Failure Independent of Age
A post-hoc analysis from GALACTIC-HF supports the potential efficacy of omecamtiv mecarbil irrespective of age, including in patients with severe heart failure. Of the 8,232 patients included in the analysis, patients were on average 64.5 years old, with 54.5% aged ≥65 years. At baseline, patients aged ≥65 years were more likely to be women, have atrial fibrillation or flutter, have worse
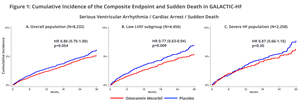
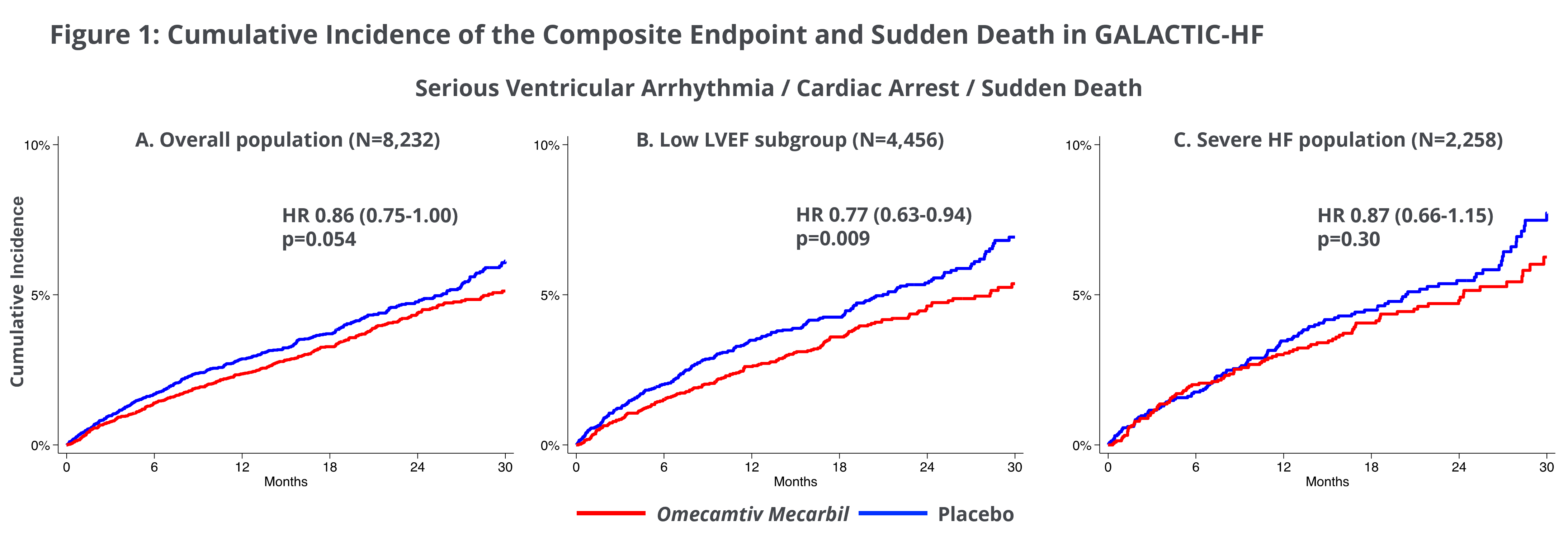
Ventricular Arrythmias Associated with Adverse Clinical Outcomes in Patients with Heart Failure and Reduced Ejection Fraction; Treatment with Omecamtiv Mecarbil Reduces Risk of Ventricular Arrythmias in Patients with Severely Reduced LVEF
A second post-hoc analysis from GALACTIC-HF investigated clinical consequences of ventricular arrhythmias (VA) in the patients studied in GALACTIC-HF. Of the 8,232 patients enrolled in GALACTIC-HF, following an occurrence of
About Omecamtiv Mecarbil
Omecamtiv mecarbil is an investigational, selective, small molecule cardiac myosin activator, the first of a novel class of myotropes1 designed to directly target the contractile mechanisms of the heart, binding to and recruiting more cardiac myosin heads to interact with actin during systole. Omecamtiv mecarbil is designed to increase the number of active actin-myosin cross bridges during each cardiac cycle and consequently augment the impaired contractility that is associated with heart failure with reduced ejection fraction (HFrEF). Preclinical research has shown that omecamtiv mecarbil increases cardiac contractility without increasing intracellular myocyte calcium concentrations or myocardial oxygen consumption.2-4
The development program for omecamtiv mecarbil assessed its potential for the treatment of HFrEF. Positive results from GALACTIC-HF, the first Phase 3 clinical trial of omecamtiv mecarbil demonstrated a statistically significant effect of treatment with omecamtiv mecarbil to reduce risk of the primary composite endpoint of cardiovascular (CV) death or heart failure events (heart failure hospitalization and other urgent treatment for heart failure) compared to placebo in patients treated with standard of care. No reduction in the secondary endpoint of time to CV death was observed. In general, the overall rates of myocardial ischemia, ventricular arrhythmias and death were similar between treatment and placebo groups. Adverse events and treatment discontinuation of study drug were balanced between treatment arms. The magnitude of the treatment effect in a pre-specified subgroup of more than 4,000 patients with heart failure with severely reduced ejection fraction (<30%) was observed to be greater than in the overall drug treated population of GALACTIC-HF. Omecamtiv mecarbil will be the subject of COMET-HF (Confirmation of Omecamtiv Mecarbil Efficacy Trial in Heart Failure), a confirmatory Phase 3 clinical trial in patients with symptomatic heart failure with severely reduced ejection fraction, expected to start in Q4 2024.
About Heart Failure with Severely Reduced Ejection Fraction
Heart failure is a grievous condition that affects more than 64 million people worldwide5 about half of whom have reduced left ventricular function.6,7 It is the leading cause of hospitalization and readmission in people age 65 and older.8,9 By 2029 is it estimated that 2.8 million people in the
About
For additional information about
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act").
CYTOKINETICS® and the C-shaped logo are registered trademarks of
Contact:
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- Psotka MA, Gottlieb SS, Francis GS et al. Cardiac Calcitropes, Myotropes, and Mitotropes. JACC. 2019; 73:2345-53.
- Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J. et al. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil.
Nat Commun . 2017;8:190. - Shen YT, Malik FI, Zhao X, et al. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010; 3: 522-27.
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011 Mar 18;331(6023):1439-43.
- James et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators.
Lancet 2018; 392: 1789–858. - Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Roger VL. Epidemiology of Heart Failure.
Circulation Research . 2013;113:646-659, originally published August 29, 2013. doi: 10.1161/CIRCRESAHA.113.300268. - Kilgore M, Patel HK, Kielhorn A, et al. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017; 10: 63-70.
- Taylor C J, Ordóñez-Mena J M, Roalfe A K, et al. Trends in survival after a diagnosis of heart failure in the
United Kingdom 2000-2017: population based cohort study. BMJ 2019; 364:l223 doi:10.1136/bmj.l223 - Greene SJ, Bauersachs J, Brugts JJ, et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. JACC. 2023 Jan 31;81(4):413-424. doi:10.1016/j.jacc.2022.11.023. PMID: 36697141.
- Extrapolated from Desai NR, Butler J, Binder G, et al. Prevalence and Excess Risk of Hospitalization in Heart Failure with Reduced Ejection Fraction. Poster presented at: Heart Failure Society of America (HFSA) Annual
Scientific Meeting ; 2022Sep 30-Oct 3 ;Washington, DC . - Carnicelli AP, Clare RM, Hofmann P, et al. Clinical trajectory of patients with a worsening heart failure event and reduced ventricular ejection fraction. Am Heart J. 2022 Mar; 245:110-116. doi: 10.1016/j.ahj.2021.12.003. Epub 2021 Dec 18. PMID: 34932997.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ee1006b4-fa47-4800-8929-5cdb837d546e

Source: Cytokinetics, Incorporated