Cytokinetics Announces Primary Results From SEQUOIA-HCM Presented in Late Breaking Clinical Trial Session at the European Society of Cardiology Heart Failure 2024 Congress and Published in the New England Journal of Medicine
Statistically Significant and Clinically Meaningful Improvements Observed in Primary Efficacy Endpoint and All Secondary Endpoints; Results Consistent Across All Prespecified Subgroups
Rapid and Sustained Improvements Observed in Symptoms and Function;
No Treatment Interruptions Due to Low LVEF
Company to Host Investor Event and Webcast Today at
SEQUOIA-HCM enrolled 282 patients with obstructive HCM. The baseline characteristics of patients in SEQUOIA-HCM were well-matched between treatment groups and consistent with a symptomatic patient population that had high resting and post-Valsalva gradients (mean [SD]; 55.1 [29.6] and 83.1 [32.3] mmHg, respectively) reflective of substantial burden of disease. Background therapies consisted of beta-blockers (61.3%), calcium channel blockers (28.7%), and disopyramide (12.8%), with combination background therapies permitted.
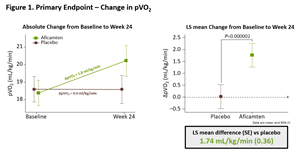
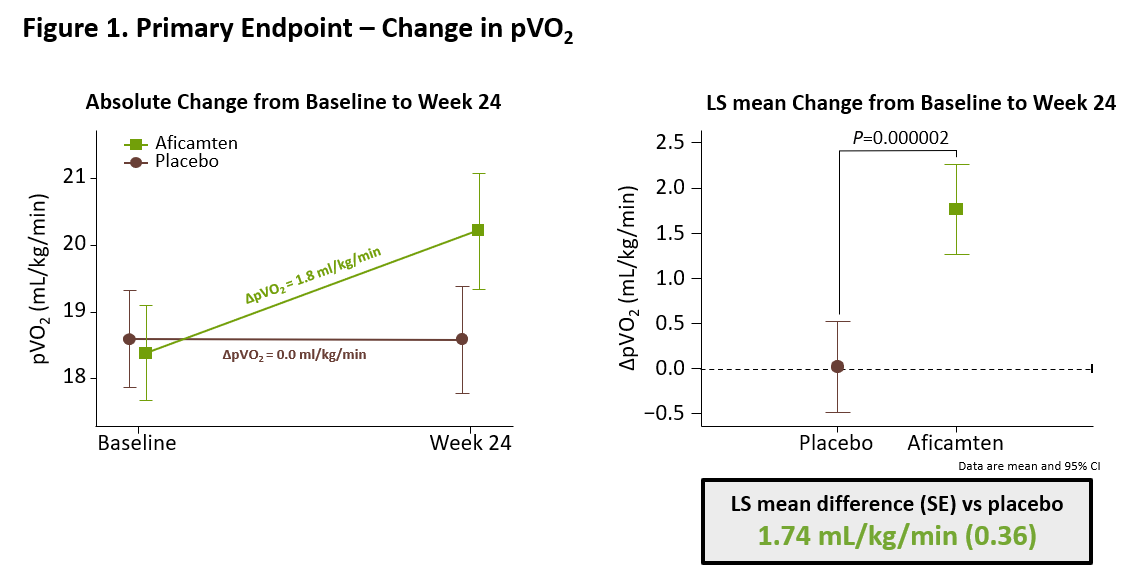
The results from SEQUOIA-HCM showed that treatment with aficamten for 24 weeks significantly improved exercise capacity compared to placebo, increasing peak oxygen uptake (pVO2) measured by cardiopulmonary exercise testing (CPET) by 1.8 ml/kg/min compared to baseline in patients treated with aficamten versus 0.0 ml/kg/min in patients treated with placebo (least square mean (LSM) difference [95% CI] of 1.74 mL/kg/min [1.04 - 2.44]; p=0.000002) (Figure 1).
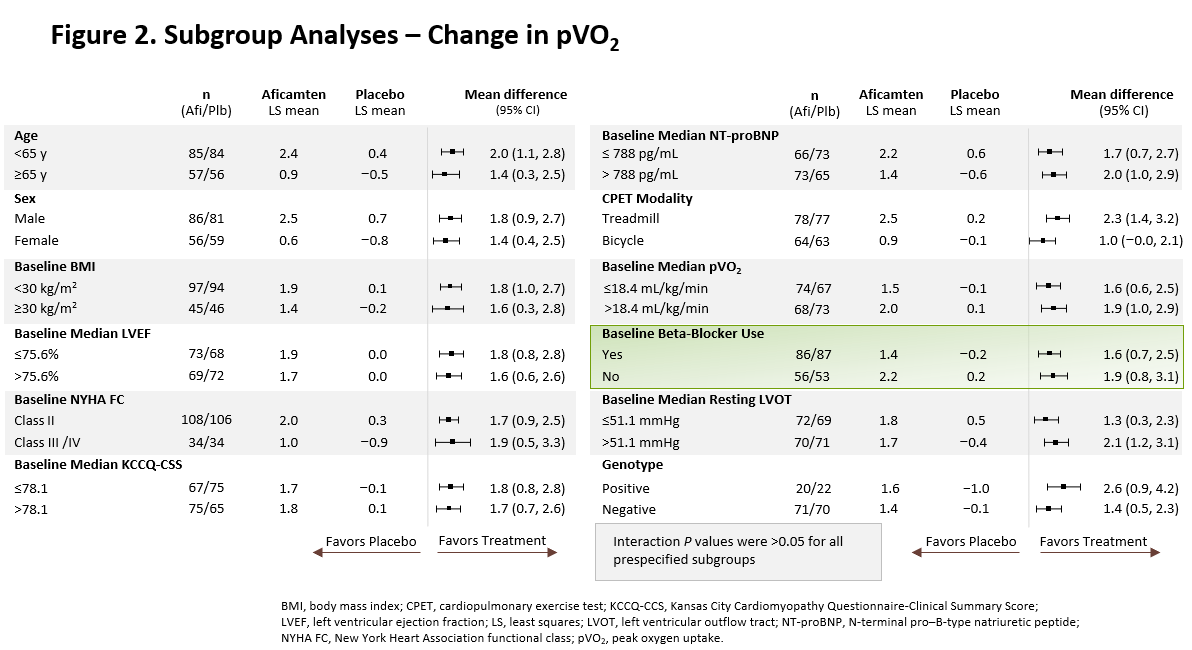
The treatment effect of aficamten was consistent across all prespecified subgroups, including age, sex, patient baseline characteristics, and in patients receiving or not receiving background beta-blocker therapy (Figure 2).
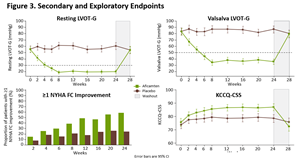
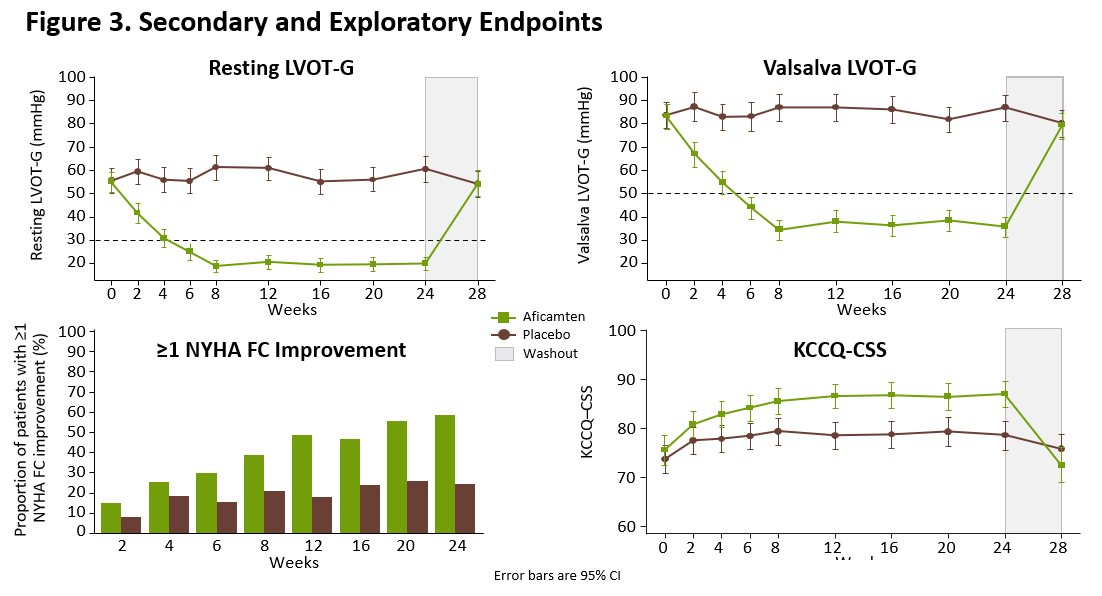
Statistically significant improvements were observed in all 10 prespecified secondary endpoints, with functional and symptomatic improvements occurring within two weeks of initiating treatment with aficamten and sustained throughout the treatment period. Compared to baseline, at Week 24 patients treated with aficamten experienced significant improvements in post-Valsalva left ventricular outflow tract gradient (LVOT-G) with an LSM difference of -50 mmHg (p<0.0001) versus placebo. Aficamten also substantially reduced the burden of symptoms compared with placebo, with a significant improvement observed in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) (LSM difference = 7 points; p<0.0001) and with 34% of patients experiencing ≥1 class improvement in
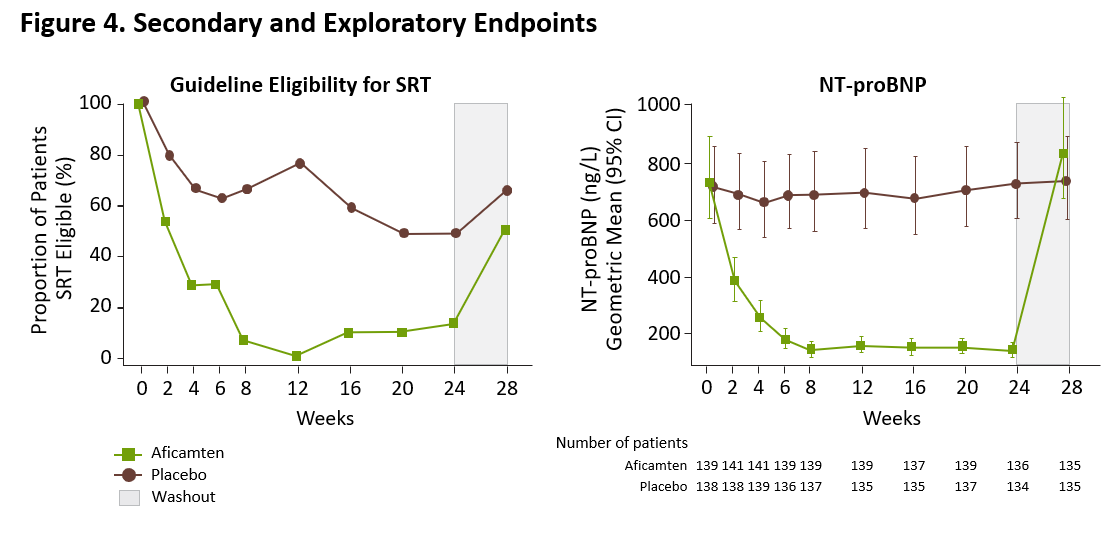
The prespecified exploratory responder analysis in SEQUOIA-HCM showed that treatment with aficamten improved both exercise capacity and symptoms, with 60 (42%) of 142 patients treated with aficamten achieving the composite responder endpoint of (1) ≥1.5 mL/kg/min increase in pVO2 and ≥1 NYHA Functional Class improvement, or (2) ≥3.0 mL/kg/min increase in pVO2 and no worsening of NYHA Functional Class, compared to 19 (14%) of 140 patients treated with placebo, equating to a placebo-corrected difference of 28.7% (95% CI, 18.8, 38.6; p<0.0001).
“The results from SEQUOIA-HCM demonstrate that treatment with aficamten is associated with statistically significant, rapid and sustained improvements in exercise capacity, symptoms, cardiac function and cardiac biomarkers in patients with obstructive HCM,” said Fady I. Malik, M.D., Ph.D., Cytokinetics’ Executive Vice President of Research & Development. “With no treatment interruptions due to low LVEF, aficamten appeared safe and well-tolerated. We believe these results are strongly supportive of the potential approval of aficamten, and we look forward to submitting regulatory filings in both the
“These results are remarkable, not only because of the magnitude of effect on clinical measures of disease, but also for the consistency observed across patient subgroups, including patients taking beta-blockers at baseline, which in real-world practice represents a large portion of patients with obstructive HCM,” said
Aficamten was well-tolerated in SEQUOIA-HCM with an adverse event profile comparable to placebo. Treatment emergent serious adverse events occurred in 5.6% and 9.3% of patients on aficamten and placebo, respectively. Core echocardiographic left ventricular ejection fraction (LVEF) was observed to be <50% in 5 patients (3.5%) on aficamten compared to 1 patient (0.7%) on placebo. One of the 5 patients on aficamten with low LVEF had LVEF <40% following infection with COVID-19 but did not interrupt treatment as the site-read LVEF remained greater than 40% and the patient did not have symptoms of heart failure due to systolic dysfunction. Overall, there were no instances of worsening heart failure or treatment interruptions due to low LVEF.
Investor Event and Webcast Information
About Aficamten
Aficamten is an investigational selective, small molecule cardiac myosin inhibitor discovered following an extensive chemical optimization program that was conducted with careful attention to therapeutic index and pharmacokinetic properties and as may translate into next-in-class potential in clinical development if approved. Aficamten was designed to reduce the number of active actin-myosin cross bridges during each cardiac cycle and consequently suppress the myocardial hypercontractility that is associated with hypertrophic cardiomyopathy (HCM). In preclinical models, aficamten reduced myocardial contractility by binding directly to cardiac myosin at a distinct and selective allosteric binding site, thereby preventing myosin from entering a force producing state.
About the Broad Phase 3 Clinical Trials Program for Aficamten
The development program for aficamten is assessing its potential as a treatment that improves exercise capacity and relieves symptoms in patients with HCM as well as its potential long-term effects on cardiac structure and function.
Aficamten was evaluated in SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM), the pivotal Phase 3 clinical trial in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). Aficamten is currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, CEDAR-HCM, a trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. Aficamten received Breakthrough Therapy Designation for the treatment of symptomatic obstructive HCM from the
About Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a disease in which the heart muscle (myocardium) becomes abnormally thick (hypertrophied). The thickening of cardiac muscle leads to the inside of the left ventricle becoming smaller and stiffer, and thus the ventricle becomes less able to relax and fill with blood. This ultimately limits the heart’s pumping function, resulting in reduced exercise capacity and symptoms including chest pain, dizziness, shortness of breath, or fainting during physical activity. HCM is the most common monogenic inherited cardiovascular disorder, with approximately 280,000 patients diagnosed in the
About
For additional information about
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act’s Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements express or implied relating to the properties or potential benefits of aficamten or any of our other drug candidates, our ability to obtain regulatory approval for aficamten for the treatment of obstructive hypertrophic cardiomyopathy or any other indication from FDA or any other regulatory body in
CYTOKINETICS® and the C-shaped logo are registered trademarks of
Contact:
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- Maron, MS, et al. Aficamten for Symptomatic Obstructive Hypertrophic Cardiomyopathy. N Engl J Med. DOI: 10.1056/NEJMoa2401424
- CVrg: Heart Failure 2020-2029, p 44; Maron et al. 2013 DOI: 10.1016/S0140-6736(12)60397-3; Maron et al 2018 10.1056/NEJMra1710575
Symphony Health 2016-2021 Patient Claims Data DoF;- Maron MS, Hellawell JL, Lucove JC,
Farzaneh-Far R , Olivotto I. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy inthe United States . Am J Cardiol. 2016; 15;117(10):1651-1654. - Gersh, B.J., Maron, B.J., Bonow, R.O., Dearani, J.A., Fifer, M.A., Link, M.S., et al. 2011 ACCF/AHA guidelines for the diagnosis and treatment of hypertrophic cardiomyopathy. A report of the
American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines.Journal of the American College of Cardiology and Circulation , 58, e212-260. - Hong Y, Su WW,
Li X. Risk factors of sudden cardiac death in hypertrophic cardiomyopathy. Current Opinion in Cardiology. 2022Jan 1 ;37(1):15-21
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/00e6ed31-7232-4315-a63c-32a6f9be2361
https://www.globenewswire.com/NewsRoom/AttachmentNg/2e58d188-fc45-4530-9c66-8a908c532ff1
https://www.globenewswire.com/NewsRoom/AttachmentNg/da6ac93c-54bc-4f54-b10b-385b8b552129
https://www.globenewswire.com/NewsRoom/AttachmentNg/5f2f3370-b173-4184-a068-6c87c2935b7f

Figure 1. Primary Endpoint Change in pVO2
Figure 1. Primary Endpoint Change in pVO2
Figure 2. Subgroup Analyses - Change in pVO2
Figure 2. Subgroup Analyses - Change in pVO2
Figure 3. Secondary and Exploratory Endpoints
Figure 3. Secondary and Exploratory Endpoints
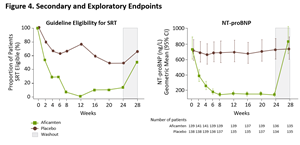
Figure 4. Secondary and Exploratory Endpoints
Figure 4. Secondary and Exploratory Endpoints
Source: Cytokinetics, Incorporated