Cytokinetics Announces Data From Phase 1 Study of CK-4021586
Phase 2 Clinical Trial in Patients with Heart Failure with Preserved Ejection Fraction Expected to Begin in Q4 2024
“The results from this Phase 1 study replicate pre-clinical findings that show CK-586 directly reduces cardiac contractility at the level of the sarcomere. Importantly, CK-586 was observed to have a shallow and predictable PK/PD relationship and half-life that enables a once-daily fixed dosing regimen in patients with HFpEF,” said
Phase 1 Design and Key Findings
The primary objective of this Phase 1 double-blind randomized, placebo-controlled, single and multiple ascending dose clinical study was to evaluate the safety, tolerability and PK of CK-586 when administered orally to healthy participants. The study design included seven single ascending dose cohorts (10 mg to 600 mg) comprised of 10 participants each, and two multiple-dose cohorts (100 and 200 mg once daily) comprised of 10 participants each.
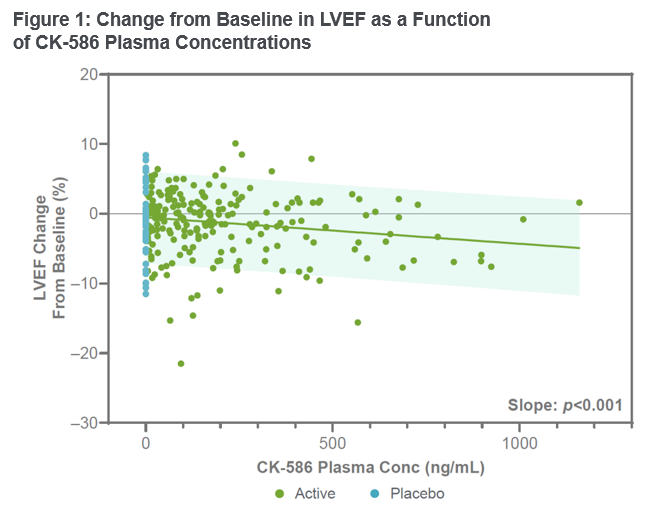
This study data demonstrated that CK-586 was safe and well tolerated in healthy participants. No serious adverse events were observed and the stopping criteria for the study were not met. The half-life of CK-586 was observed to be in the range of 14 to 17 hours. CK-586 demonstrated dose-linearity without a change in half-life over a wide range of exposures, with steady state appearing evident within seven days of dosing. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) decreased from baseline in an exposure-dependent manner, and the pharmacokinetic/pharmacodynamic (PK/PD) relationship appeared shallow and predictable (Figure 1). At the highest single dose of 600 mg, the mean decrease in LVEF was <5%. These results demonstrate pharmacologic properties that may enable once-daily fixed-dose administration in the future. Preparations for a Phase 2 clinical trial in patients with heart failure with preserved ejection fraction (HFpEF) are underway and the trial is expected to begin in Q4 2024.
About CK-4021586 (CK-586)
CK-4021586 (CK-586) is a novel, selective, oral, small molecule cardiac myosin inhibitor designed to reduce the hypercontractility associated with heart failure with preserved ejection fraction (HFpEF). CK-586 selectively inhibits the ATPase of intact cardiac myosin but does not inhibit the ATPase of subfragment-1 of myosin (S1) as does aficamten, a cardiac myosin inhibitor also developed by the Company. Unlike aficamten, the inhibitory effect of CK-586 requires the presence of the regulatory light chain (RLC) of myosin in the context of the intact myosin dimer (heavy meromyosin or HMM). In preclinical models, CK-586 reduced cardiac hypercontractility by decreasing the number of active myosin cross-bridges during cardiac contraction thereby reducing the contractile force, without effect on calcium transients. In engineered human HCM heart tissues, CK-586 demonstrated a shallow force-concentration response and improved lusitropy. Lending support for investigating this mechanism of action in HFpEF, a subset of patients with HFpEF resemble patients with non-obstructive hypertrophic cardiomyopathy (HCM) in that those patients have higher ejection fractions, thickened walls of their heart, elevated biomarkers, and symptoms of heart failure. Data from a Phase 2 clinical trial of aficamten in patients with non-obstructive HCM show that aficamten was well tolerated, improved patient reported outcomes (
About Heart Failure with Preserved Ejection Fraction
Heart failure is a grievous condition that affects more than 64 million people worldwide.1 Approximately 6.7 million Americans have heart failure, which is expected to increase to over 8.5 million Americans by 2030.2 Approximately half of patients with heart failure have heart failure with preserved ejection fraction (HFpEF)3, and the prevalence of HFpEF is increasing.2,4 A subset of HFpEF patients with hypercontractility, ventricular hypertrophy, elevated biomarkers and symptoms of heart failure may benefit from treatment with a cardiac sarcomere inhibitor. Approximately 75% of patients with HFpEF will die within five years of initial hospitalization, and 84% will be rehospitalized.2 Despite broad use of standard treatments and advances in care, the prognosis for patients with heart failure is poor.5
About
For additional information about
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act").
CYTOKINETICS® and the C-shaped logo are registered trademarks of
Contact:
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- James et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators.
Lancet 2018; 392: 1789–858. - Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K, Fonarow GC, Heidenreich P, Ho JE, Hsich E, Ibrahim NE, Jones LM, Khan SS, Khazanie P, Koelling T, Krumholz HM, Khush KK, Lee C, Morris AA, Page RL 2nd, Pandey A, Piano MR, Stehlik J, Stevenson LW, Teerlink JR, Vaduganathan M, Ziaeian B; Writing Committee Members. Heart Failure Epidemiology and Outcomes Statistics: A Report of the
Heart Failure Society of America . J Card Fail. 2023 Oct;29(10):1412-1451. doi: 10.1016/j.cardfail.2023.07.006. Epub 2023 Sep 26. PMID: 37797885; PMCID: PMC10864030. - Dunlay SM, Roger VL,
Weston SA , Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012 Nov;5(6):720-6. doi: 10.1161/CIRCHEARTFAILURE.111.966366. Epub 2012 Aug 30. PMID: 22936826; PMCID: PMC3661289. - Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Jhund PS, MacIntyre K, Simpson CR, et al. Long-Term Trends in First Hospitalization for Heart Failure and Subsequent Survival Between 1986 and 2003. Circulation. 2009;119:515-523.
A graph accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/baf4dfcb-789b-45f8-9ae2-e670fb20f2a8

Source: Cytokinetics, Incorporated